Difference Between Validation Calibration And Qualification In Pharma

Difference Between Validation Calibration And Qualification In Pharma There is often confusion surrounding the terms validation, calibration, and qualification within the pharmaceutical industry. let’s explore their differences with explanatory examples. 1. qualification: qualification is the process of planning, conducting, and documenting test results performed on equipment to confirm its operational capability. The action of proving and documenting that any process, procedure or method actually and consistently leads to the expected results. it can better understand that the validation is a documented evidence to and done to prove the consistency of the expected results of any process, procedure or method. related: validation in pharmaceutical.

Difference Between Validation Calibration And Qualification In Pharma What is the difference between validation, qualification, and calibration? calibration ensures measurement accuracy, qualification verifies equipment readiness, and validation ensures process consistency. In summary, calibration focuses on ensuring the accuracy of measuring instruments, validation ensures the reliability and accuracy of methods and systems, and qualification verifies the suitability and compliance of equipment, facilities, and systems used in the pharmaceutical industry. This article explores the definitions, purposes, examples, regulatory requirements, and key differences between calibration, validation, and qualification in the pharmaceutical industry. Installation qualification (iq) is performed for new equipment, and operation qualification (oq) and performance qualification (pq) monitor the day to day running of that equipment. calibration, meanwhile, is vital in proving and maintaining the legitimacy of qualification and validation.

Difference Between Validation Calibration And Qualification In Pharma This article explores the definitions, purposes, examples, regulatory requirements, and key differences between calibration, validation, and qualification in the pharmaceutical industry. Installation qualification (iq) is performed for new equipment, and operation qualification (oq) and performance qualification (pq) monitor the day to day running of that equipment. calibration, meanwhile, is vital in proving and maintaining the legitimacy of qualification and validation. In short, regular calibration allows pharmaceutical companies to have confidence in their results which they can record, monitor and control. you can read also: difference between calibration and validation. Calibration is the process of adjusting or verifying the accuracy of an instrument, while qualification is the process of ensuring that an instrument or system meets specific requirements. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. validation is the collection and assessment of data from process design to commercial phase, which establishes objective evidence that a process can consistently deliver a quality product. Both qualification and validation are integral to ensuring that pharmaceutical products are consistently produced and meet required quality standards. however, understanding the differences between the two is crucial for regulatory compliance and maintaining product quality.
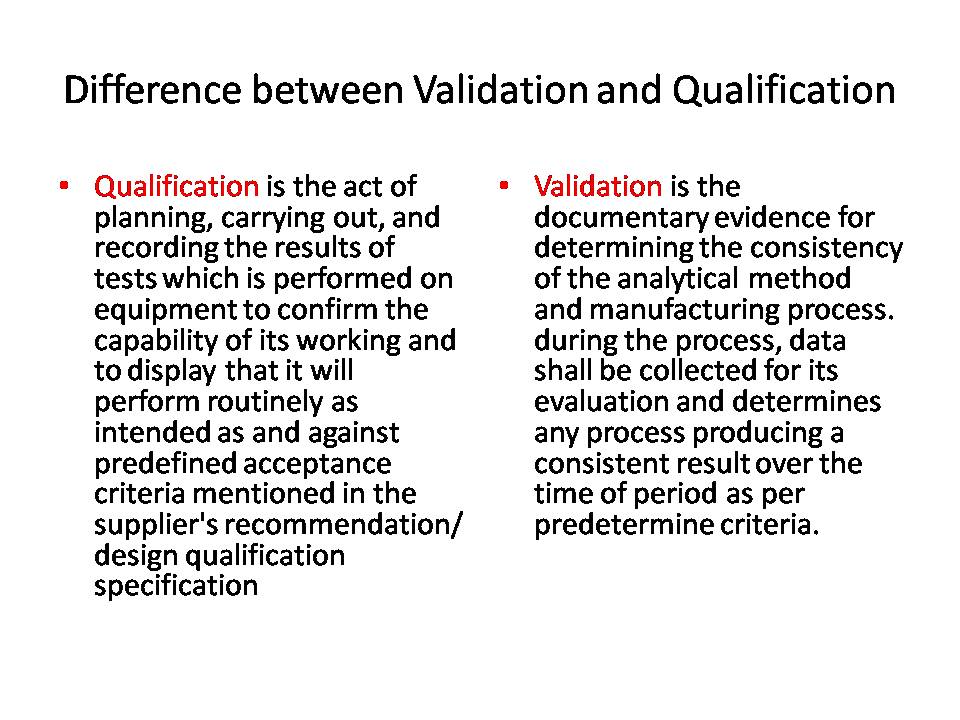
Difference Between Validation Calibration And Qualification In Pharma In short, regular calibration allows pharmaceutical companies to have confidence in their results which they can record, monitor and control. you can read also: difference between calibration and validation. Calibration is the process of adjusting or verifying the accuracy of an instrument, while qualification is the process of ensuring that an instrument or system meets specific requirements. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. validation is the collection and assessment of data from process design to commercial phase, which establishes objective evidence that a process can consistently deliver a quality product. Both qualification and validation are integral to ensuring that pharmaceutical products are consistently produced and meet required quality standards. however, understanding the differences between the two is crucial for regulatory compliance and maintaining product quality.

Difference Between Validation Calibration And Qualification In Pharma Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. validation is the collection and assessment of data from process design to commercial phase, which establishes objective evidence that a process can consistently deliver a quality product. Both qualification and validation are integral to ensuring that pharmaceutical products are consistently produced and meet required quality standards. however, understanding the differences between the two is crucial for regulatory compliance and maintaining product quality.
Comments are closed.